
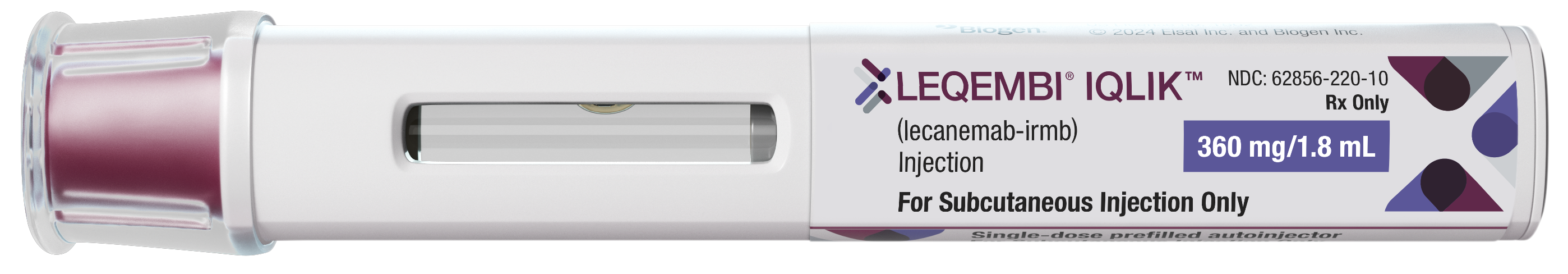
NOW FDA
APPROVED!
Learn more
about the option to switch to
LEQEMBI IQLIK after 18 months of infusions.
One of TIME'S BEST INVENTIONS 2025. From TIME © 2025 TIME USA LLC.
All Rights Reserved. Used under license.


